The Boma
Welcome to ‘The Boma’—a new podcast about livestock in the developing world—the cattle, camels, sheep, goats, pigs and poultry—that provide billions of people with nutrition, income, resources and livelihoods. How can small scale livestock systems be sustainable, as well as profitable? How can they help protect the environment? Do they harm or enhance human health? Check out The Boma to hear diverse perspectives on some of the hottest topics debated today and dive deep into the best and latest scientific research on livestock and development. ****** The Boma is hosted by Global Livestock Advocacy for Development (GLAD), a project of the International Livestock Research Institute (ILRI), and funded by the Bill & Melinda Gates Foundation.
The Boma
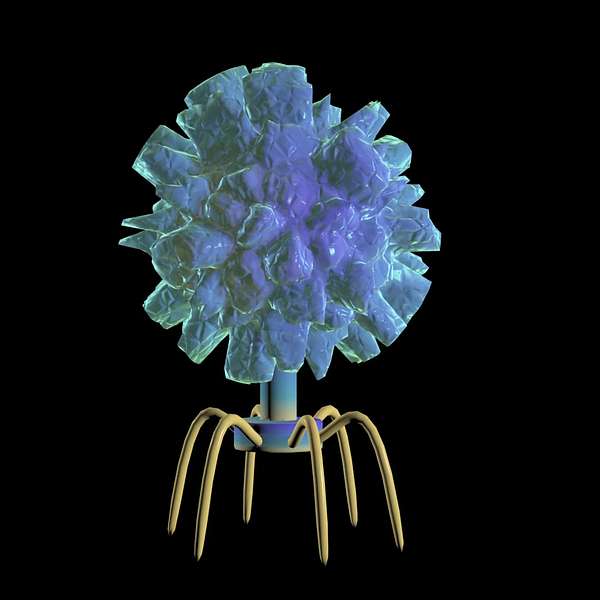
Phages - Bad for bacteria, good for livestock farmers
Livestock farmers use antibiotics to treat infections in their animals, and may also use them as a preventative. But overuse of antibiotics can create 'superbugs' - antimicrobial-resistant (AMR) bacteria which threaten human lives and wellbeing, as well as those of livestock animals.
Presenters Brenda Coromina and Elliot Carleton explore one approach that ILRI scientists are taking to combat the AMR problem - phages. These 'bacteria-eating' viruses, which naturally exist in the environment, are being studied by ILRI scientists to develop an alternative treatment to antibiotics. They hear from 'phage hunters' Angela Makumi and Nicholas Svitek about how phages work, what makes them different from antibiotics, and what it will take to make phage therapy a reality.
Could phages become our future weapon of choice against bacteria?
Read more:
Phages: The viruses that offer a sustainable alternative to antibiotic treatment in livestock
Brenda Welcome Back to The Boma.
A podcast from the International Livestock Research Institute where we discuss how sustainable livestock is contributing to development efforts in the Global South.
My name is Brenda Coromina.
Elliot: And I’m Elliot Carleton.
Brenda: Today, we are talking about bacteriophages, and the name should tell you a thing or two about what they do.
The word Phage comes from a Greek root which means to “eat” or to “devour”.
Elliot: So, what you’re saying is that phages essentially eat bacteria?
Brenda: Yes — More precisely, they’re viruses which infect and destroy bacteria.
Nicholas: In a way phages are bad viruses for the bacteria themselves, but they are good viruses for us or animals.
Brenda: That’s Dr Nicholas Svitek, one of the two ILRI scientists we’ll be hearing from today.
He and Dr. Angela Makumi are both working on Phage research.
They’re going to talk to us about the frightening topic of antimicrobial resistance, — more commonly known as AMR — and how they’re using phages to combat AMR bacteria.
Elliot: So, why would we want to use viruses to kill bacteria? Can’t we just use things like soap, chemicals, medicines…
Brenda: Sure, but those things are not always as helpful as they seem.
Consider antibiotics – they save lives by killing bacteria. But overusing antibiotics is what causes AMR to develop.
And it’s a serious problem once you can’t kill harmful microbes like bacteria. Every year, over 700,000 people die because of AMR ‘superbugs’.
Elliot: Ok, so how do you kill a superbug??
Brenda: Well, let’s talk about one way that scientists are looking into it - phages.
Music break
Brenda: When it comes to livestock farming, AMR is a widespread but complicated issue.
Antibiotics aren’t just used in intensive factory farming in the Global North, or even just to treat animals when they’re sick—they’re used by farmers in the Global South, who are worried about their livelihoods and their food security.
Angela: I would say like livestock farming is relied upon by a lot of farmers to provide the basic needs in the household. And because of this, competition in sustainable livelihood, a lot of farmers tend to use antibiotics just for either treatment or prevention or just for growth promotion. And this is just to help them sell the product to the consumer. Because remember, this is also they need this source of income. So they use a lot of antibiotics.
Elliot: That was Dr. Angela Makumi, a post-doctoral fellow at ILRI specializing in microbiology, molecular biology, and bacteriophage biology.
She and Nicholas Svitek, another microbiologist at ILRI, have been analysing bacteria found in livestock farms in Kenya.
Nicholas: What we have observed in our research is that a lot of the bacterial isolates that we have collected from the environment are, I mean, I would say at least a third of them are multidrug resistant to several antibiotics. The few times I've been to the farms, we have observed that antibiotics are quite often used. They're usually delivered to the feed or water. So sometimes you have some like, I don't know, some vitamins delivered through the water, but they contain antibiotics. And sometimes they will treat their chicken not knowing what's causing the infection. So they will use antibiotics at large just to treat or prevent infections.
Brenda: The main point here is that AMR isn’t just limited to bacteria which infect livestock. And it’s not limited by geography.
It matters for the entire world, not just for developing countries.
Elliot: Right. Humans can also pick up AMR bacteria.
Angela: So of course, we are looking at the high rate of resistance that we are creating and we’re creating this, the so called superbugs.
And also when you look at it, the lack of wastes management at farm level, also can instigate that process of antibiotic resistance to humans, meaning that if you don't treat your waste, if you don't manage your waste well from the farms, this waste ends up into the water system. And maybe the same people are using the same water, waterpoint. And so you're carrying this resistant bacteria to humans, to livestock, to the environment.
Nowadays with a global travel, resistance is not only an isolated incident. So for example, if I have resistant bugs and I travel to Europe or to Australia or to any other continent, it means that I have the likelihood of spreading this bug into the environment.
Elliot: The problem is that farmers can use the same antibiotics that hospitals would use, too.
Angela: Take a situation where there's a farmer who is having an animal, and this animal has an infection, and he's been giving too many antibiotics. And when you look at how the close proximity between the farmer and the animal, they tend to be in close proximity every day. So that means the farmer can get this resistant bacteria. And if he gets sick, the problem is, most of the antibiotics that are used also by farmers are clinically relevant antibiotics. There are those that if he gets an infection, then even the clinically relevant antibiotics will not be able to help the farmer. And now we're looking at a very bad case where you have resistance that is now in humans.
Brenda: Situations like this are already happening, and there are wider, more hidden costs too.
Nicholas: If the animal is infected and sick, then you might not be able to treat it. So then it might die. So there would be a food security issue to humans who rely on animal products from the from these livestock species. And also it can lead to economic security issues for the farmers selling these products.
Brenda: So, farmers might overuse antibiotics in their livestock because they want them to be healthy and survive infections.
Healthy, thriving animals lead to more meat, milk and eggs — meaning better food security and economic security.
But because of AMR, the opposite could happen.
An expert report predicts that by 2030, 24 million more people could be forced into extreme poverty because of AMR.
Elliot: And so this is where phages come in, which entails fighting bacteria with viruses.
Brenda: Right.
Think of phages as highly evolved, natural nanomachines that kill bacteria with exceptional specificity.
That sounds pretty dramatic, but for Angela, she first became interested in phages by considering how humans interact with livestock.
Angela:
I have always been passionate about agriculture, especially in terms of farm to fork, meaning that a lot of thought has to be put in how we interact with livestock in the farm, and what we end up consuming in our own tables for dinner, or for lunch. So for this reason, I became interested in phages, because they have been used as an ancient cure and a therapeutic. And they're also appealing because there's now a higher tail on organic choice. And there's also increased to natural antimicrobial and alternatives.
I'm very keen on animal and human health, and how the interface between the two happens. And I'm also interested in terms of antimicrobial resistance that could happen within this interface, and what would be the long term repercussions. So coming up with a phage product is also important because this can create sustainability for the African market.
Elliot: OK, but, how do phages destroy bacteria, exactly?
Nicholas: Phages are basically viruses. What is particular about phages is that they will inject their genetic material and then it will start generating several copies of the phages. And once there's enough copies of the phage, then they will lyse the bacterial cell, and by doing this, they will kill the bacteria and release more phages in the environment.
So in a way phages are bad viruses for the bacteria themselves, but they are good viruses for us or animals.
Brenda: So essentially, phages infect bacteria, multiply, the bacteria bursts open and is destroyed, and then the phages disperse to find other bacteria to infect.
If this all still sounds a little alarming, Angela assures us that they’re not harmful to humans.
Angela: So like just a game, like when you're playing a game of Lego, phages use specific receptors that can only be found on the bacterial cell. And by using this receptors, they use them specifically to bind and gain entry to the bacteria, and that therefore infecting and killing the bacteria. But however, they do not infect or kill mammalian cells.
Elliot: That sounds amazing! So when can livestock farmers expect to get their hands on phage technology?
Angela: I would say phage therapy, it's still at its infancy, and we still have a long way to go. But some great strides have been made in isolating and characterization. But however, the next leap would be to move from lab based research into practical field based research.
Nicholas: But also phage could be used, could be sprayed, for instance over the skin of animals if they have an infection, or a wound. So the most common way of using phages would be through the oral route.
Elliot: So, phages can potentially be administered in a variety of ways…
But what happens if farmers overdo it? Won’t we see phage-resistant bacteria?
Brenda: Not according to Angela.
Bacteria become resistant to antibiotics when the dose isn’t high enough to kill all of them. Those that survive become resistant and keep reproducing. But as Angela explains, phages aren’t like antibiotics, and they evolve too quickly for resistance to be a problem.
Angela: We should remember that antibiotics are chemical molecules that targets specific molecular machinery in the bacteria. And therefore when this machinery evolves, the bacteria is no longer sensitive to the antibiotic. But when we were looking at phages, phages are very, very specific, and they use a narrow. Specificity means they are also narrow, so they only use one part. They don't use like a whole machinery. They can maybe use one patch to infect the cell. Because we are living in kind of an evolutionary race between the bacteria and the phage, they will always co evolve together. So, if one bacteria mutates, and becomes insensitive to the phage, the phages also mutate because remember, the phage needs the bacteria to multiply. So the phage, it's always like a race between the bacteria and the phage. So if bacteria evolves, the phage will also evolve. So you'll always have phage for a mutant bacteria.
Brenda: OK, I now want to take us back to the more practical side of things.
If farmers are going to be able to use phages -- how much more will it cost than something like antibiotics?
Angela: So the recommendation is that phages are isolated from their specific regions, and this is because of their bacterial hosts. Like the strains that will be circulating in the US or in Europe would be completely different from those circulating in Africa. However, we lack the infrastructure or the personnel who are well trained in isolating phages. In terms of making it into a product, the cost is lesser compared to the antibiotic, but the process could be actually laborious, but the process is actually can, is also shorter. Because an antibiotic is a chemical agent, that you have to prove that it has not have any effect in the animal or human.
Through collaborations and funding and proper infrastructure, you're looking at maybe one to two years, and also collaborating with companies who can make a product like dry, so that when you're making a product, you don't have to make it in such a way. Like local farmers don't, may or may not be able to have like a freezer or a fridge. So you have to make a product that is for local people and can be stored at room temperature but the same time the phage still remains effective.
Elliot: I imagine that storage can still present its own set of issues for farmers.
Brenda: Actually, storage is one of the things that make phages inaccessible to many people in developing countries.
At the moment, they need to be refrigerated, but scientists are researching alternative ways to keep them.
Nicholas: Okay, so for our day to day lab work, I mean, we keep them at four degrees in the refrigerator. And for our stocks, we keep them at minus 80 in the freezer, that goes to many degrees Celsius. But then, for, if we eventually develop a phage therapy or a cocktail, we would investigate how we can either lyophilize or what other people uses a technology called spray drying. So it's just basically to remove the humidity from the samples and have like a powder like you know, instant coffee, but these are phages, that you can resuspend in a buffer before delivering these phages to the animals. So by doing this, we would increase the survivability of phages at room temperature, hopefully.
So having those freeze dried, or spray dried phages would remove the need of a cold chain to deliver the phage to the field, and just keep them at room temperature in remote villages, or where there's no access to a refrigerator or electricity.
Elliot: Well, now I’d like to know what the first phage treatment might be.
Brenda: You might have heard of Salmonella?
Elliot: Isn’t that one of the bacteria that can cause food poisoning?
Brenda: Right. Dr. Svitek is currently leading a team of microbiologists to use phages to treat Salmonella, and especially AMR Salmonella, in chickens.
Nicholas: Our project is trying to develop phage therapy as a viable option to treat or prevent some of the infections in chicken in Kenya, but eventually this could go and we could expand this to include salmonella from other countries or include other pathogens of poultry or even pathogens that affect other livestock species.
Elliot: I guess one last question that comes to mind is whether phage therapy will ever replace antibiotics in the future, if all the right conditions are met to make it accessible for everyone.
Brenda: I was thinking that as well.
At this point, when it comes to bacteria, it might sound like antibiotics are the bad guy and phages should just completely replace them.
But that’s not necessarily the case.
Nicholas: I don't think they will ever replace completely antibiotics. And the reason is that five phages might not be the best strategy to treat some infections for instance, to treat bacteria that are intracellular.
And then I mean there's also other alternatives to antibiotics. So there's like vaccines, immunomodulators, probiotics, also phage derived products, antimicrobial peptides. So all of this can be used as an arsenal if you will, to have different strategies to replace antibiotics. But I think antibiotics will still be around. What we need this better control of antibiotic use, a better education and several researchers have suggested that we can, a good strategy that could work is to combine antibiotics and phages, that together synergize, to be more efficient. So studies have shown that if you use an antibiotic in an infection, it reduces bacterial load. If you use phages alone, it does the same effect. But if you combine both together, you have 1000 fold reduction as compared to using these alone. So that could be a strategy to combine phages and antibiotics, and perhaps even using less antibiotics then normally required.
Elliot: That’s really interesting that antibiotics and phages can actually work together.
And what I like is that this research into AMR and phages is not about one narrow bit of science. It affects food security, human health, and the environment.
Brenda: Right.
And phages are just one of the ways that scientists are working to combat this issue. They are relatively inexpensive, and they can potentially treat infections in livestock while reducing the problem of overusing antibiotics.
And that's a great place to leave off for today. Thank you so much to Dr. Angela Makumi and Dr. Nicholas Svitek for taking the time to share their work with us.
Elliot: And thank you to our listeners for joining us today.
If you enjoyed today’s episode, we hope that you will leave us a review, and please don’t forget to subscribe! We will catch you next time on The Boma.
I’m Elliot Carleton.
Brenda: And I’m Brenda Coromina